Abstract
Introduction: Natural killer (NK) cells, as the major subset of innate lymphocytes, produce proinflammatory cytokines and mediate anti-tumor cytotoxicity 1-3. Since NK cells are not limited by clonotypic receptors, they can be utilized in cell therapies against a broad spectrum of malignancies. Irrespective of the clinical potentials, the transcriptional regulation of the development and functions of human NK cells is far from understood. This knowledge gap presents significant limitations in formulating successful clinical applications. Human NK cells develop from common lymphoid progenitors and progress through NK progenitors (NKPs), immature NK (iNKs, CD56bright), mature NK (mNK, CD56dim), and terminally mature NK cells (tNKs) 4. One of the master regulators of human NK cell development is GATA2 5-7.
Patients with GATA2 haploinsufficiency specifically lose CD56bright NK cells, with or without a reduced number of CD56dim NK cells 6. In our current study, we define the role of GATA2 in the development and functions of NK cells derived from patients with GATA2 mutation.
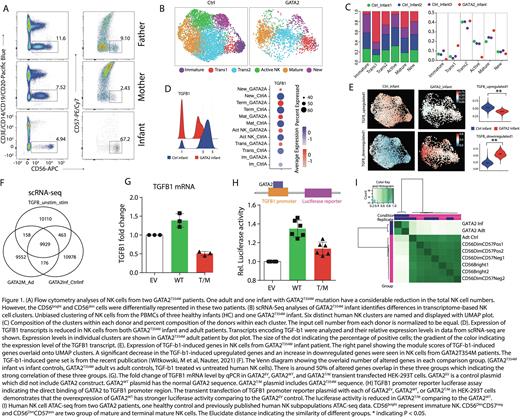
Results: The PBMCs from three GATA2T354M donors and the corresponding healthy controls (HC) were obtained from the Children's Hospital of Wisconsin and Cincinnati Children Hospital. Two of three donors are related family members. The flow cytometry reveals the adult GATA2T354M donor had reduced NK cell number, and the absence of CD56bright NK cells (Fig. 1 A). However, the infant GATA2T354M had reduced total NK cells, especially NK cell is predominantly presented as CD56bright NK cells compared to controls (Fig.1 A). To address NK cell phenotypical alteration of GATA2T354M, we performed single-cell RNA-seq (scRNA-seq). Our data indicated that GATA2T354M patients have reduced immature NK cells populations (Fig.1 B, C) as typical expected NK cell phenotype alteration. We also found that the hemostatic activated NK cell population, defined as the active NK, also significantly decreased in both of GATA2T354M patients (Fig. 1 B, C).
To explore the mechanism of NK cell alterations in GATA2 patient, we compared the transcriptomic differences in scRNA-seq data. We identify that TGF-b1 (Fig. 1 D), its targets and suppressors are significantly reduced in GATA2T354M patients compared to controls. TGF-b1 is an essential cytokine in maintaining NK cell immaturity [8,9]. We applied the gene sets that was upregulated and downregulated by TGF-b1 treated vs untreated human NK cell. We found that TGF-b1-upregulated gene set (182 genes) is significantly reduced in GATA2T354M patients; TGF-b1-downregulated genes (254 genes) is significantly increased (Fig. 1 E). The Venn diagram showed that around 50% of differential expression genes (DEGs) of GATA2T354M patients vs controls overlap with the DEGs between TGF-b1-treated vs untreated NK cell (Fig. 1 F). These alterations strongly indicate the existence of GATA2-TGF-b1 axis in human NK cells, which is not known before.
To further validate the GATA2-TGF-b1 axis ex-vivo, we transfected GATA2EV, GATA2WT, and GATA2T354M plasmid into HEK-293T cells to quantify the TGFB1 mRNA level by qPCR. The results reveal that TGFB1 mRNA is increased in GATA2WT group, but it is decreased in the GATA2T354M group (Fig. 1 G). To provide the evidence that GATA2 directly binds to TGFB1 and regulates its expression, we performed the TGFB1 promoter reporter assay. GATA2WT or GATA2T354M plasmid with the TGFB1 reporter plasmid are transfected together into HEK-293T cells. The relative luciferase activity demonstrates that GATA2 indeed binds to the TGFB1 promoter region as the stronger luciferase activity in the GATA2WT comparing to GATA2EV group. However, it decreases in GATA2T354M group comparing to GATA2WT group (Fig. 1 H). The GATA2T354M patient NK cell ATAC-seq data indicates the significantly altered epigenetic profile (Fig. 1 I). In addition, the TGFB1 locus in GATA2 patients is less open comparing to controls. We are performing Cut&Tag to identify GATA2 regulating network on human NK cell development and provide additional evidence that TGFB1 gene is a direct target of GATA2.
Conclusion: Through this study, we define the role of GATA2 via TGF-b1 in human NK cell development and functions, provide direct evidence for findings of NK cell deficiency among GATA2T354M patient. Importantly, we will determine the unique developmental and functional states of human NK cells and the regulating transcriptional programs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal